
There is a widespread belief claiming that it’s dangerous to reheat spinach. You’ve probably heard something like “You should NEVER reheat spinach.” After that, you’ve probably started feeling threatened to eat your spinach meal leftovers.
You’ve probably Googled or encountered questions like: “Is it dangerous to reheat spinach? Is it safe to reheat spinach? Is it unhealthy to reheat spinach? Can I eat spinach the next day? Does reheating spinach cause cancer?” etc.
And the results: numerous articles indicate that warming spinach is harmful.
Is this, however, true?
The short answer is that reheated spinach is safe to consume, but only under certain conditions. Let’s dig deeper!

Why do people believe that reheating spinach is dangerous?
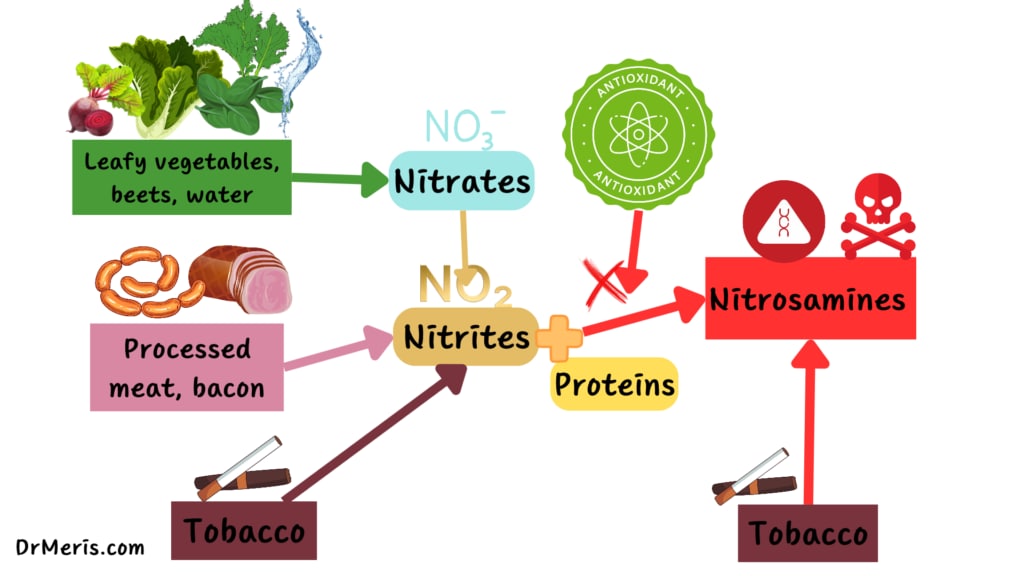
Nitrate, nitrites, and nitrosamines are behind this myth. What are these?
Nitrates and nitrites are essential compounds. Spinach contains high levels of nitrates.
Nitrates are made up of a single nitrogen atom and three oxygen atoms. When humans consume naturally occurring nitrates, the digestive tract degrades them, removing one oxygen atom and forming a nitrite.
Nitrates are further broken down when they are subjected to extreme heat or the microorganisms in our gut. It becomes nitric oxide if it loses one more oxygen molecule to our gut flora, which is excellent for our bodies since it helps convey crucial signals that help regulate blood pressure.
So far, everything is fine, but nitrates can be dangerous if they produce nitrosamines.
Nitrosamines can occur when nitrates or nitrites are cooked at high temperatures.1
This is where the myth began. But wait don’t leave it here and keep reading!
Most people’s diets contain nitrates, nitrites, and nitrosamines. Nitrates and nitrites exist naturally in fruits and vegetables, which are considered an important part of a healthy diet due to strong evidence of cancer-fighting properties.2
Cancer risk is the most serious adverse impact of nitrate and nitrite intake because of the creation of nitrosamine compounds, a high number of which are regarded as carcinogenic.3
Nitrosamines come in a variety of forms, and many of them can increase the risk of cancer.4 these substances are major carcinogens in tobacco. They can be found in unburned tobacco leaves because they are created during processing, such as curing, fermentation, and aging.5
Nitrosamines are formed through chemical interactions between nitrates, nitrites, and other proteins. N-nitrosodimethylamine (NDMA) is one of the most common nitrosamines found in our diet.3
NDMA is a carcinogen that can cause malignant tumors in a range of animal tissues, including the liver, lung, and stomach.6
High quantities of sodium nitrite can be found in bacon, hot dogs, and processed meat. They also include a lot of protein, which is made up of amino acids. When exposed to intense heat, this combination generates the ideal circumstances for the formation of nitrosamines.7
Food additives nitrates and nitrites were linked to an increased risk of breast and prostate cancer.7
Based on studies related to colorectal and stomach cancer, the International Agency for Research on Cancer (IARC) determined processed beef subjected to, among other things, curing to be a Group 1 carcinogen. Recent research in this area has concentrated on identifying alternatives to nitrates/nitrites in meat processing.8
However, due to the multifunctional character of nitrate, no substance has been discovered that can completely replace the roles of nitrites or nitrates. The good effects of nitrites/nitrates in cured meat products are related to color enhancement, the development of cured meat flavor, the antibacterial role, and the antioxidative effect.1
Let’s get back to spinach:
Spinach and other leafy plants contain nitrates that are not harmful to our health. But why?
Bacteria convert nitrates to nitrite under specific conditions and temperatures, which can then be transformed into carcinogenic nitrosamines in the body. The conversion of nitrate to nitrite occurs only at high temperatures.
Cooking vegetables, however, is less likely to produce nitrosamines. People rarely cook vegetables at very high heat, and they don’t contain large amounts of protein, which contributes to the formation of nitrosamine.
Even if the temperatures are high enough, the time it takes to cook or heat spinach is brief, leaving bacteria little time to convert nitrate.9
According to Jeanne de Vries, associate professor of Dietary Assessment, the belief that reheating spinach is harmful extends back to a time when not every household had a refrigerator.9
So as long as you store leftover spinach in the fridge in the meantime, you should be fine.
As I have stated at the outset of this article, nitrogen oxide, which is created from nitrate, can lower blood pressure, making it beneficial to your cardiovascular health.
Vegetables also include antioxidants, which limit the conversion of nitrates. However, unlike vegetables, meat lacks antioxidants, which is why the Netherlands Nutrition Centre advises against eating processed meat regularly due to its link to stomach and bowel cancer.9
How about the nitrates converted into nitrites in our bodies?
In that regard, the amount of nitrate we consume through green vegetables is relatively small. However, it can pose issues for newborns.
A high level of nitrite in the bloodstream binds to red blood cells and inhibits them from supplying oxygen, resulting in Blue Baby Syndrome (also called methemoglobinemia). In babies, this process is more difficult to reverse than in adults.
Depending on the severity of their disease, affected infants have a strange blue-gray skin color and may become irritable or lethargic. If the condition is not diagnosed and treated promptly, it can quickly progress to coma and death. In babies, this process is more difficult to reverse than in adults.10
Blue Baby Syndrome is primarily caused by contaminated drinking water and is especially common in developing countries where populations in rural areas get their drinking water from wells.10
What are the foods that contain nitrosamines?
Many food varieties contain nitrosamines, including cured meat products, processed seafood, cocoa, beer, and other alcoholic beverages. Meat and meat products are the major food groups that contribute to nitrosamine exposure.9
Processed vegetables, cereals, milk and dairy products, and fermented, pickled, and spicy foods may also contain nitrosamines.9
To sum up
Nitrates contained in spinach and other green leafy vegetables are not hazardous to your health. Nitrogen oxide, produced from nitrate, can lower blood pressure, which is advantageous to your cardiovascular health.
Under certain conditions and temperatures, bacteria convert nitrates to nitrite, which the body converts to carcinogenic nitrosamines. Only at high temperatures does nitrate convert to nitrite.
Cooking vegetables produces fewer nitrosamines. Even at high temperatures, the time it takes to cook or heat up spinach is short, giving bacteria little time to convert nitrate. Furthermore, veggies include antioxidants, which prevent nitrate conversion.
So as long as you keep any remaining spinach in the fridge, you should be good.

How to store spinach?
Once cooked and cooled, transfer your spinach to an airtight container and store it in the refrigerator. Cooked spinach will keep in the fridge for up to 5 days if properly preserved.
Fresh spinach can be difficult to keep. If there is any moisture in the leaves, they will wilt and rot, releasing a terrible odor and encouraging all the leaves around them to do the same.
Use a paper towel before placing fresh spinach in the fridge to keep your spinach fresh for as long as possible. This will help the absorption of any moisture that may be present.
It’s also preferable to store your spinach in a container rather than a bag because breaking the leaves may cause them to wilt and rot.
How to freeze spinach?
Frozen spinach is a great option if you have more spinach than you can consume in a few days. Freezing your leafy greens will protect their nutritious value better than keeping them in the fridge.
The leaves will no longer be crisp and fresh once defrosted, thus this is not an excellent technique to preserve salad greens. However, if you intend to prepare your spinach in the future, cleaning, blanching, and freezing is an excellent year-round solution for garden-fresh spinach.
If you’re working with fresh spinach from the garden, you should wash the leaves several times to remove all the dirt. If your spinach is supermarket fresh, you may be able to skip this step if the box specifies that it has been pre-washed.
Blanching your spinach simply involves heating it for a brief length of time, usually 2 minutes, and then immediately placing it in an ice bath to stop the cooking process. You want to kill all hazardous germs while not cooking the spinach.
It’s critical to remove all of the water from the spinach before freezing it. If you don’t thoroughly dry your spinach before freezing it, it will become mushy when it defrosts. You can blot with a cloth or paper towel or use a salad spinner.
The most convenient way to store spinach is in a freezer-safe Ziploc bag. The goal is to pack in as many leaves as possible to decrease the space air can get between them. If you’re using a bag, you can push out any remaining air as you seal it.
References
1. Shakil, M. H. et al. Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review. Foods (Basel, Switzerland) 11, (2022). http://dx.doi.org/10.3390/foods11213355
2. Bradbury, K. E., Appleby, P. N. & Key, T. J. Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). The American journal of clinical nutrition 100 Suppl 1, 394s-398s, (2014). http://dx.doi.org/10.3945/ajcn.113.071357
3. Tricker, A. R. N-nitroso compounds and man: sources of exposure, endogenous formation and occurrence in body fluids. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP) 6, 226-268, (1997).
4. Brown, J. L. N-Nitrosamines. Occupational medicine (Philadelphia, Pa.) 14, 839-848, (1999).
5. Yalcin, E. & de la Monte, S. Tobacco nitrosamines as culprits in disease: mechanisms reviewed. Journal of physiology and biochemistry 72, 107-120, (2016). http://dx.doi.org/10.1007/s13105-016-0465-9
6. Tricker, A. R. & Preussmann, R. Carcinogenic N-nitrosamines in the diet: occurrence, formation, mechanisms and carcinogenic potential. Mutation research 259, 277-289, (1991). http://dx.doi.org/10.1016/0165-1218(91)90123-4
7. Scanlan, R. A. Formation and occurrence of nitrosamines in food. Cancer research 43, 2435s-2440s, (1983).
8. Karwowska, M. & Kononiuk, A. Nitrates/Nitrites in Food-Risk for Nitrosative Stress and Benefits. Antioxidants (Basel, Switzerland) 9, (2020). http://dx.doi.org/10.3390/antiox9030241
10. Knobeloch, L., Salna, B., Hogan, A., Postle, J. & Anderson, H. Blue babies and nitrate-contaminated well water. Environmental health perspectives 108, 675-678, (2000). http://dx.doi.org/10.1289/ehp.00108675
Sign Up for Our Email List
Get our latest articles, healthy recipes, tips, and exclusive deals delivered straight to your inbox with our newsletter.
We won't send you spam. Unsubscribe at any time.









Sustain the excellent work and producing in the group!